The Challenge: Health Inequity is Perpetuated by an Exclusionary Approach to Clinical Research & Development
The purpose of the pharmaceutical industry—namely pharma/biopharma, med-tech/bio-techs and nonprofit product development partnerships (PDPs)—is to innovate to better serve the vast and increasingly complex health and well being needs of our global population. Boosting access to life-saving and life-enhancing technologies has long been a focus of the health community, and industry has increasingly been making efforts to bolster access to existing products and therapeutics through a range of downstream investments (see FSG’s Competing by Saving Lives series for examples).
However, even when physical access is obtained to medicines and therapies, it is becoming increasingly understood that the products themselves are often not researched and developed with the needs of all potential end-users (patients) in-mind. Paradoxically, in the process of obtaining the most rigorous efficacy and safety data to enable registration of new medicines to be released to market, many populations who are the most vulnerable and will likely benefit from the therapy more than others have been systematically excluded from the clinical R&D process. For the sub-set of diseases where clinical R&D is moved forward, data is often generated in male animals in pre-clinical stages, while clinical trials are often conducted in (healthy) men who are often white and living in high-income countries.
While this current paradigm offers scientific rigor, the data collected pre-registration does not necessarily represent those who may ultimately get the drug prescribed to them or excludes populations entirely, such as pregnant and lactating women or people living in certain places (e.g., Africa). Without more intentional consideration of the diversity of needs across the entire R&D value chain, large sways of the population will continue to be underserved and health inequities perpetuated.
The Solution: Accelerating Inclusive R&D by Industry Players
A paradigm shift is needed from exclusionary practices to inclusive research and development. By embracing a more intentionally inclusive approach to clinical R&D, industry specifically can be more effective in realizing its purpose. Inclusive R&D strategies will better address societal needs in ways that may also improve competitive advantage and/or help companies and their partners make explicit the trade-offs required to sustainably advance more equitable approaches to R&D, and collaboratively go beyond these choices. To identify and discuss these opportunities and trade-offs, companies need to have the appropriate metrics and measurement systems in place. This data enables dialogue internally within the company and then in discussion with key stakeholders how to jointly define strategies to solve for trade-offs, including those that are less commercially viable.
Across industry, many pharmaceutical companies and nonprofit development actors have in recent years begun to make the commitment to becoming more inclusive. In March 2023, the U.S. Food and Drug Administration announced a new requirement for researchers and companies to submit a plan for ensuring diversity among clinical trial participants (see also Advancing Health Equity: How Companies Can Build on the FDA’s Requirement for Clinical Trial Diversity).
However, some are making more progress than others. While there are a few emerging industry examples, initiatives are fragmented across different disease verticals and/or geographies or focused on specific sub-populations and are often not publicly accessible.
An Industry Blueprint for Inclusive R&D Would Help Accelerate Mind-Set Shifts and Unlock Innovation
Scientific narratives—and power—hold the paradigm of exclusion in place. These narratives shape everything from what research is conducted, what data is collected (or not) and then stored in databases and used in modelling tools even prior to clinical trial, to the clinical protocols and criteria for participation and beyond. All of which ultimately drive systematic exclusion of diverse populations, particularly marginalized groups, from fully benefiting from—and participating in—clinical research.
Take selection criteria for participation in clinical trials. It is now being argued that the bar is set so high that one academic describes the process as “somehow looking for Olympic athletics” and many patients most in need are simply not eligible to participate. We argue that many of these patients are likely experiencing an unequitable burden of disease compared to the general population. This unequitable burden of disease may be a consequence of sustained experiences of poor social conditions that increase the risk of poor health and more complex health needs over time. For instance, a lack of access to housing in cities with clean air increases risk of respiratory illness and other chronic disease. A lack of personal autonomy to enforce the practice of safe-sex or use of contraceptives places women at risk of sexual infection or unplanned pregnancy. A lack of access to clean and safe public transport undermines access to a range of services and to get to school and work. The root causes of many of these experiences are anchored in structural determinants of health, such as social classism, sexism, racism, ableism, and so forth.
Not only are these barriers driving poorer health outcomes. Without being factored into the arc of clinical R&D process from beginning to end, the therapeutic outputs from industry and beyond will continue to fall short of delivering the greatest potential benefit with confidence for all.
This poses a considerable challenge to the pharma industry as it undermines its purpose. Implications are far reaching for industry both in terms of competitive differentiation and costs. By not fully considering the entire universe of unmet needs, it may be true that large opportunities for competitive advantage remain untapped, such as opening up new segments of payer markets. On the cost side, insufficient data slows the entire R&D process down. Up to 86% of oncology trials in the U.S. fail to complete recruitment on time at great expense. Regulators are demanding additional trial arms to be carried out as a result of sub-populations being excluded from the original protocol—such as in the case of the HIV prevention drug, Descovy—likely adding greater cost than if included in the original design. This was all prior to the onset of the COVID-19 pandemic, where confidence and trust in the industry was being tested through the roll-out of the COVID-19 vaccine, with high rates of vaccine hesitancy, confusion around safety, and growing awareness of the limits of clinical trial diversity (e.g., only 2% of clinical trials took place in Africa before COVID-19 and drugs now offered for the disease are tested in non-Africans). Furthermore, new technologies, such as precision medicine, raise concerns around further exacerbating disparities.
These trends are demanding that the pharmaceutical industry must evolve in order to retain its license to innovate and operate. We argue that in addition, these shifts also offer potential business opportunities for differentiation and competitive advantage alongside deeper societal impact.
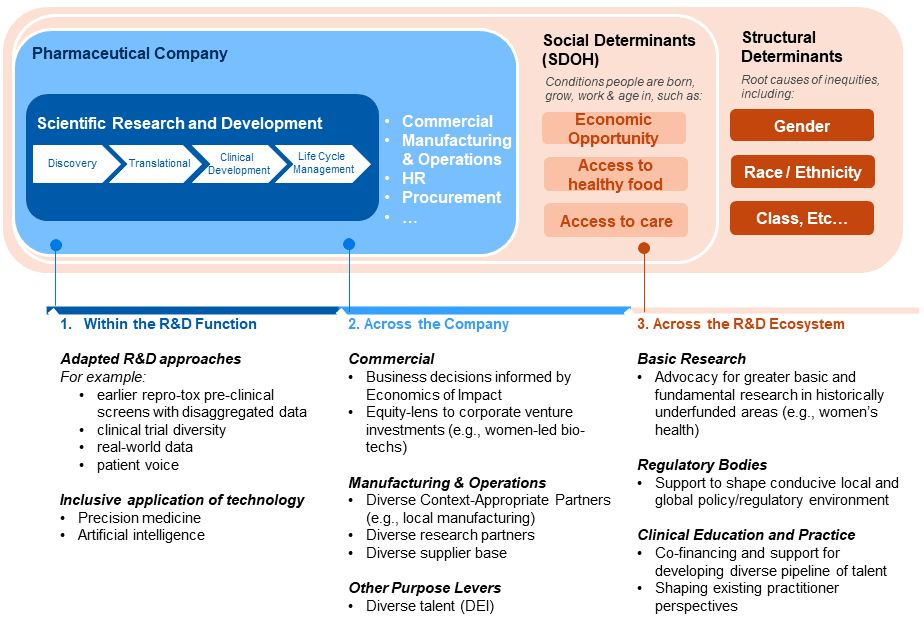
A fresh approach and reframing of R&D to be more intentionally inclusive and holistic is needed for industry. Such an approach must be supported by a set of strategies anchored in the industry’s unique value-add, expertise and assets, alongside “asks” from industry of other ecosystem players for enabling and accelerating change (see Figure 1).
Figure 1. Example Industry Purpose Levers for Inclusive R&D (non-exhaustive)
Within the Pharmaceutical Company: Drawing upon our health equity work with clients in the sector, there are a range of emerging options and approaches for adapting and enhancing the industry approach to inclusive R&D. There are opportunities to made adjustments within the R&D function, such as revising the clinical development approach to collect data earlier so to enable better decision-making, such the proposal for the inclusion of pregnant and lactating women in anti-malaria research (see also, “Re-Orientating anti-malarial drug development to better serve pregnant women”). Opportunities also lie in other areas of the firm, including corporate venture investments and local manufacturing. Building out a deeper understanding of the benefits for society and business, including understanding how to navigate costs and risks to deliver on these different strategies and options will be important, given that issues such as liability and reputational risk are common barriers to action.
Across the R&D Ecosystem: It is important to recognize that while the pharma industry is a key player in adopting and advancing inclusive R&D practices, it is one stakeholder in a broad R&D ecosystem. This ecosystem includes patients, researchers, ethics committees, regulators, public research bodies, and public and private payers. Decision-makers across these groups offer different levers and incentives for supporting (or mandating) industry to transition to inclusive R&D. Regulators are already shifting towards inclusion. Identifying opportunities for partnership and collaboration with other players in the broader ecosystem, including academia, civil society, and other sectors such as tech will be an important element of the work. Envisioning a future state for inclusive R&D, for instance, that lays out how current practice must adapt and shift could help organize more intentionally coordinated action across players overall.
A Call to Action: Taking Steps to Advance Industry Best Practice in Inclusive R&D
Accelerating the paradigm shift from exclusion to inclusion is essential for delivering more equitable health outcomes. We believe a more expansive set of guidance and best practices drawn from industry examples alongside a coordinated ask to ecosystem players would be valuable in driving this change beyond clinical trial diversity. We would love to hear about how companies are working across the levels and invite you to reach out to us through this quick form: